Many protozoa live in the human body. Many of them are pathogens. Our story is about ten, the most. The review is based on both historical and recent publications.
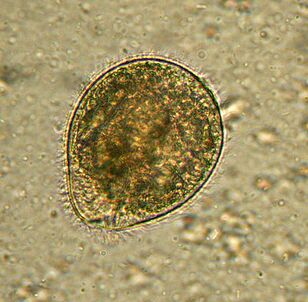
Largest. BalantidiumBalantidium coli
The largest protozoan is a human parasite, and the only cilia in this company. Its dimensions range from 30 to 150 microns and its width from 25 to 120 microns. By comparison, the length of the malarial plasmodium at the largest stage is about 15 microns and is several times smaller than the balantidium of the intestinal cells between which the cilia live. An elephant in a china shop.
Distributedwherever pigs are - their main carriers. It usually lives in the submucosa of the colon, although it also occurs in the pulmonary epithelium in humans.B. colifeeds on bacteria, food particles, fragments of the host's epithelium. In animals, the infection is asymptomatic. People can develop severe diarrhea with bloody, mucous discharge (balantidiasis), sometimes an ulcer in the wall of the colon. He rarely dies from balantidiasis but causes chronic fatigue.
People are infected with dirty water or foods containing cysts. Human infection rates do not exceed 1%, while pigs can become infected all over the world.
Antibiotics treated with antibioticsNo drug resistance has been reported for this star.
DiscoveredMalstem by Swedish scientist in 1857. Today, balantidiasis is associated with tropical and subtropical areas, poverty and poor hygiene.
The very first. Oral amoebaEntamoeba gingivalis
A parasite found in the first human amoeba. A description of the amoebae appeared in 1849 in the oldest scientific journal. Ameba was found in the plaque of the tooth, so the name comes from the Latin gingivae gum.
Almost all people live in the mouths of people with sore teeth or sore gums, inhabiting gingival pockets and plaque. It feeds on epithelial cells, leukocytes, microbes and erythrocytes. It is rare in people with a healthy oral cavity.
This small protozoan, 10-35 µm in size, does not enter the environment and does not form a cyst, but is transmitted to another host through kissing, dirty dishes, or contaminated food.E. gingivalisis considered a human parasite only, but is sometimes found in captive cats, dogs, horses, and monkeys.
In the early twentieth century,E. gingivaliswas described as a pathogen of periodontal disease because it is always present in inflamed dental cells. However, its pathogenicity has not been demonstrated.
There are no known drugs that affect this amoeba.
The most common. Dysentery amoebaEntamoeba histolytica
This intestinal parasite with blood penetrates the tissues of the liver, lungs, kidneys, brain, heart, spleen, and genitals. It eats what you get: food particles, bacteria, red blood cells, leukocytes and epithelial cells.
Widespreadeverywhere, especially in the tropics. Usually people become infected by swallowing a cyst.
In moderate countries, the amoeba usually remains in the intestinal lumen and the infection is asymptomatic. In the tropics and subtropics, the pathological process often begins:E. histolyticaattacks the walls. The reasons for the transition to the pathogenic form are still unclear, but several molecular mechanisms of this have already been described. So it is clear that amoebas secrete lysing agents, break through mucus and destroy cells. It is clear that the amoeba can kill a host cell in two ways: by inducing apoptosis or simply by chewing pieces. The first method was long considered one. Otherwise, the mechanism of cellular suicide could not be determined at a record rate - in minutes. The second method was recently described by the authors as trogocytosis from the Greek "three" to chewing. It is noteworthy that the amoebas that bite the cells die immediately after their death. Others are completely able to phagocytose dead cells. It is hypothesized that biting and digestive cells differ in gene expression pattern.
The amoeba is now able to penetrate the bloodstream, liver and other organs with trochocytosis.
Amoebiasis is a deadly disease that kills about 100, 000 people each year withE. histolyticainfection.
Dysentery has a non-pathogenic twin of amoeba,E. dispar, so microscopy is not sufficient to diagnose the disease.
Healingmust be destroyed as mobileE. histolyticaand cysts.
DescribedE. histolytica, and in 1875 determined its pathogenicity in a patient with diarrhea. The Latin name for the amoeba was given in 1903 by German zoologist Fritz Schaudin.Histolyticameans tissue destructive. In 1906, the scientist died of amoebic intestinal abscess.
The most common. Intestinal lambliaGiardia lamblia (G. gastrointestinalis)
Giardia, the most common intestinal parasite, is ubiquitous. 3-7% of people in developed countries and 20-30% in developing countries are infected. That's about 300 million people.
Parasites live in the host's duodenum and bile ducts, where they float, work with flagella, and then attach to the epithelium using a sticky disk at the bottom of the cell. At 1 cm2, the epithelium adheres to a million lambs. They damage the fluff, which interferes with the absorption of nutrients, causing mucositis and diarrhea. If the disease affects the bile ducts, it is accompanied by jaundice.
Giardiasis is a disease of dirty hands, water and food. Its simplest life cycle is simple: it has an active form in the intestine and stable cysts with stool masses at the exit. For the infection, it is enough to swallow a dozen cysts, which become active again in the intestines.
The main secret of the ubiquitous presence of lambliain the variability of surface proteins. The human body fights lamblia with antibodies and is, in principle, able to develop immunity. But people living in the same area and consuming the same water become infected again and again with the descendants of their own parasites. Why? Because the transition from the active phase to the cyst and vice versa, lamblia changes the proteins for which antibodies are produced - variant-specific surface proteins. There are about 190 variants of these proteins in the genome, but only one is always present on the surface of each parasite, the translation of the others being interrupted by the mechanism of RNA interference. And change happens about every ten generations.
Treatedwith an antiprotozoal with antibacterial activity. The disease goes away in a week, but if the bile ducts are infected, relapses are possible for many years. Cysts are fought by iodination of water.
DiscoveredGiardia lambliaIn 1859, Vilém Lambl was a Czech scientist. Since then, the simplest has changed several names, and the current one was given in honor of Alfred Giar, an explorer and French parasitologist who did not describe the lamblia.
The first sketch of Giardia was made by Anthony van Leeuwenhoek, who found it in his own nervous chair. It was in 1681.
Incidentally, Giardia is also very ancient from an evolutionary point of view, coming almost directly from all its eukaryotic predecessors.
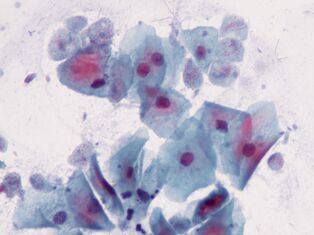
The most intimate. Trichomonas vaginalisTrichomonas vaginalis.
The simplest is sexually transmitted. It lives in the vagina and in men - in the urethra, epididymis and prostate gland - it spreads sexually or through wet washcloths. Babies can become infected by passing through the birth canal. The front end ofT. vaginalishas 4 flagella and has a relatively short undulating membrane, releasing pseudopods if necessary. The maximum size of Trichomonas is 32x12 microns.
Trichomonasis more widespreadthan the combined pathogens of chlamydia, gonorrhea, and syphilis. It affects about 10% of women, possibly more, and 1% of men. The latter data are unreliable because the parasite is more difficult to detect in men.
T. vaginalisfeeds on microorganisms, including lactic acid bacteria in the vaginal microflora, which maintain an acidic environment and thus create an optimal pH for themselves above 4. 9.
Trichomonas kills mucous cells, causing inflammation. About 15% of infected women complain of symptoms.
Treated with an antibacterial drug. As a precautionary measure, regular brushing with dilute vinegar is recommended.
Describedin 1836 by Alfred Donne, a French bacteriologist. The scientist did not understand that a pathogenic parasite was in front of him, but he determined the size, appearance, and type of movement of the simplest.
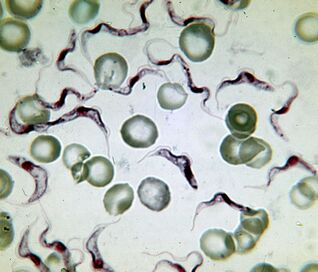
The deadliest. The pathogen of sleeping sicknessTrypanosoma brucei
The pathogen of African sleeping sickness is the deadliest protozoan. An infected person dies without treatment. The trypanosoma is an elongated flagellate 15–40 µm long. There are two subspecies that are inextricably distinguishable. Disease caused byT. brucei gambiense, lasts 2-4 years.T. brucei rhodesienseis a more virulent, transient pathogen that dies after a few months or weeks.
Widespreadin Africa, between the 15th parallel of the Southern and Northern Hemispheres, the carrier - in the natural range of blood-sucking insects of the genusGlossina(tsetse fly). Of the 31 fly species, 11 are dangerous to humans. Sleeping sickness affects the population of 37 countries in sub-Saharan Africa for 9 million km2. Up to 20, 000 people get sick every year. There are now about 500, 000 patients, 60 million at risk.
From the fly intestine,T. bruceienters the human bloodstream, from where it enters the cerebrospinal fluid and affects the nervous system. The disease begins with fever and inflammation of the lymph nodes, followed by lethargy, drowsiness, muscle paralysis, fatigue, and irreversible coma.
Parasite lethality is related to the ability of a blood-brain barrier to cross. The molecular mechanisms are not yet fully understood, but it is well known that upon entry into the brain, the parasite secretes cysteine proteases and also uses some host proteins. In the central nervous system, however, the trypanoma provides shelter from immune factors.
The first description of sleeping sickness in the upper reaches of Niger was described by the Arab scientist Ibn Khaldun (1332-1406). By the early 19th century, Europeans were already well aware of the initial signs of the disease — swelling of the lymph nodes in the back of the neck (a symptom of Winterbottom), and the slave traders paid special attention to it.
DiscoveredT. bruceiwas named after the Scottish microbiologist David Bruce, and in 1903 he first established the connection between trippanosome, fly agaric, and sleeping sickness.
Treatmentdepends on the stage of the disease, the drugs cause serious side effects. The parasite has high antigenic variability, making it impossible to create a vaccine.
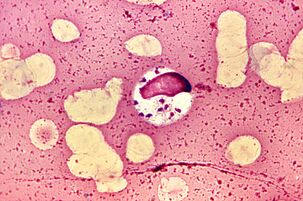
The most extravagant. LeishmaniaLeishmania donovani
Leishmanias have been named the most extravagant parasites because they live and multiply in macrophages - cells designed to kill parasites.L. donovaniis the most dangerous of them. It causes visceral leishmaniasis, commonly known as dumdum fever or kala gambling, from which almost all patients die without treatment. But survivors gain long-term immunity.
The parasite has three subspecies.L. donovani infantum(Mediterranean and Central Asian) mainly affects children, dogs are often reservoirs.L. donovani donovani(India and Bangladesh) is dangerous for adults and the elderly, there are no natural reservoirs. It can live in the blood of AmericanL. donovani chagasi(Central and South American) dogs.
L. donovani- flagellate up to 6 microns long. Humans become infected after being bitten by mosquitoes of the genusPhlebotomus, sometimes through sexual contact with babies - passing through the birth canal. Once in the blood,L. donovanipenetrates the macrophages, which carry the parasite through the internal organs. By multiplying in macrophages, the parasite kills them. The molecular mechanism of macrophage survival is quite complex.
Symptoms of the disease- fever, enlarged liver and spleen, anemia and leukopenia, which contribute to a secondary bacterial infection. Every year, 500, 000 people become ill with visceral leishmaniasis and about 40, 000 die.
Treatmentdifficult - intravenous antimony and blood transfusion.
The taxonomic affiliation ofwas determined in 1903 by the famous malaria researcher and Nobel laureate Ronald Ross of L. donovani. He owes his generic name to William Leishman and his special name to Charles Donovan, who independently discovered protozoan cells in the spleen in 1903, one in London and the other in Madras.
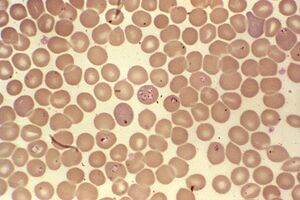
The most difficult life cycle.Babesia spp.
Babesia inBabesia in mammalian erythrocytes and in the intestines of the genusIxodescomplicated their development through transovarian transmission in addition to multistage asexual reproduction. From the intestine of a female mite, protozoan sporozoites penetrate the ovaries and infect the embryos. When the larvae of the mite hatch, the babesia passes through their salivary glands and enters the blood of the vertebrate with the first bite.
DistributedBabesia in America, Europe, and Asia. Their natural reservoir is rodents, dogs and cattle. A person has several types of infections: B. microti, B. divergens, B. duncaniandB. venatorum.
Symptoms of babesiosis are similar to malaria - recurrent fever, hemolytic anemia, enlarged spleen and liver. Most people recover spontaneously, but babesiosis can be fatal for patients with a weakened immune system.
treatment methodsare still under development while prescribing antibiotics and, in severe cases, blood transfusions.
Babesia was described by Romanian microbiologist Victor Babes, who discovered it in sick cows and sheep. He decided he was dealing with a pathogenic bacterium he namedHaematococcus bovis. Babesia was long considered an animal pathogen until it was discovered in 1957 in a Yugoslav shepherd who died of B. divergens infection.
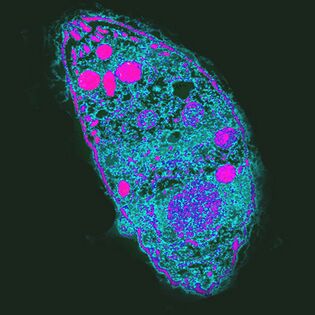
The most influential. The pathogen of toxoplasmosisToxoplasma gondii
T. gondiiis the strongest parasite because it regulates the behavior of intermediate farmers.
Distributed everywhere, unevenly distributed. For example, in France, 84% of the population is infected, and in the UK, 22%.
The life cycle of Toxoplasma consists of two stages: asexual occurs in any warm-blooded body, sexual reproduction is only possible in the epithelial cells of the cat’s gut. ToT. gondiimay stop developing, the cat must eat an infected rodent. By increasing the likelihood of the event,T. gondiiblocks rodents ’natural fear of the cat’s urine odor and makes it attractive by targeting a group of neurons in the amygdala. How he does it is unknown. One putative mechanism of action is the local immune response to infection. It changes cytokine levels, which in turn raises the levels of neuromodulators such as dopamine. Toxoplasma also influences human behavior, which manifests itself even at the population level. So in countries with a high desire for toxoplasmosis, neurotism, and uncertain avoidance, new situations are more common. It is possible thatT. gondiiinfection can lead to cultural changes.
Infectionin humans often destroys cells of the liver, lungs, brain, retina with asymptomatic but weakened immunity, causing acute or chronic toxoplasmosis. The course of the infection depends on the virulence of the strain, the condition and age of the host’s immune system - the elderly are less sensitive toT. gondii.
Toxoplasmosis can be treated with protozoal drugs.
Describedin 1908 in desert rodents. This honor goes to Charles Nicolas and Luis Manso of the Pasteur Institute in Tunisia.
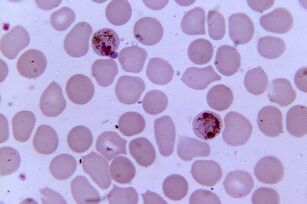
Most pathogens. Plasmodium malariaPlasmodium spp.
Plasmodium malaria is the most pathogenic parasite in humans. The number of patients with malaria can reach 300-500 million and the mortality rate of epidemics is 2 million. The disease still claims three times as many lives as armed conflict.
There are five types of Plasmodium that cause malaria in humans:Plasmodium vivax, P. falciparum, P. malariae, P. ovaleandP. knowlesi, which also affects macaques.
Distributed in the range of vectors- mosquitoesAnopheles, which require a temperature of 16-34 ° C and a relative humidity of more than 60%.
A comparison of the most virulent genome ofP. falciparumwith plasmodia with gorilla plasmodia suggests that humans were infected by their ancestors from these monkeys. The emergence of this form of Plasmodium is related to the emergence of agriculture in Africa, which has led to an increase in population density and the development of irrigation systems.Plasmodium sexual reproduction occurs in the intestines of mosquitoes, and in the human body it is an intracellular parasite that lives and multiplies in hepatocytes and erythrocytes until the cells rupture. There are 1 to 50 thousand parasites in the blood of 1 ml of patient.
The disease manifests itself as inflammation, recurrent fever and anemia, and is dangerous to the mother and fetus during pregnancy. Erythrocytes infected withP. falciparumclog the capillaries, and in severe cases, ischemia of internal organs and tissues develops.
Treatmentrequires a combination of several drugs and depends on the pathogen. Plasmodia becomes drug resistant.






































